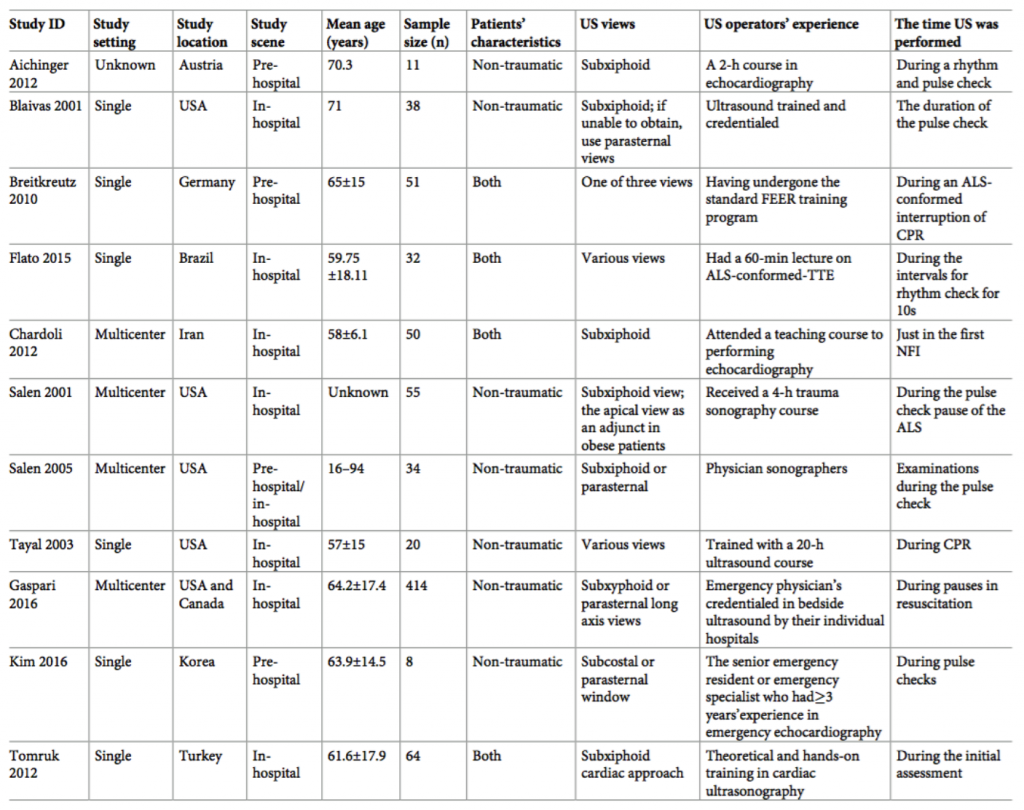
Background
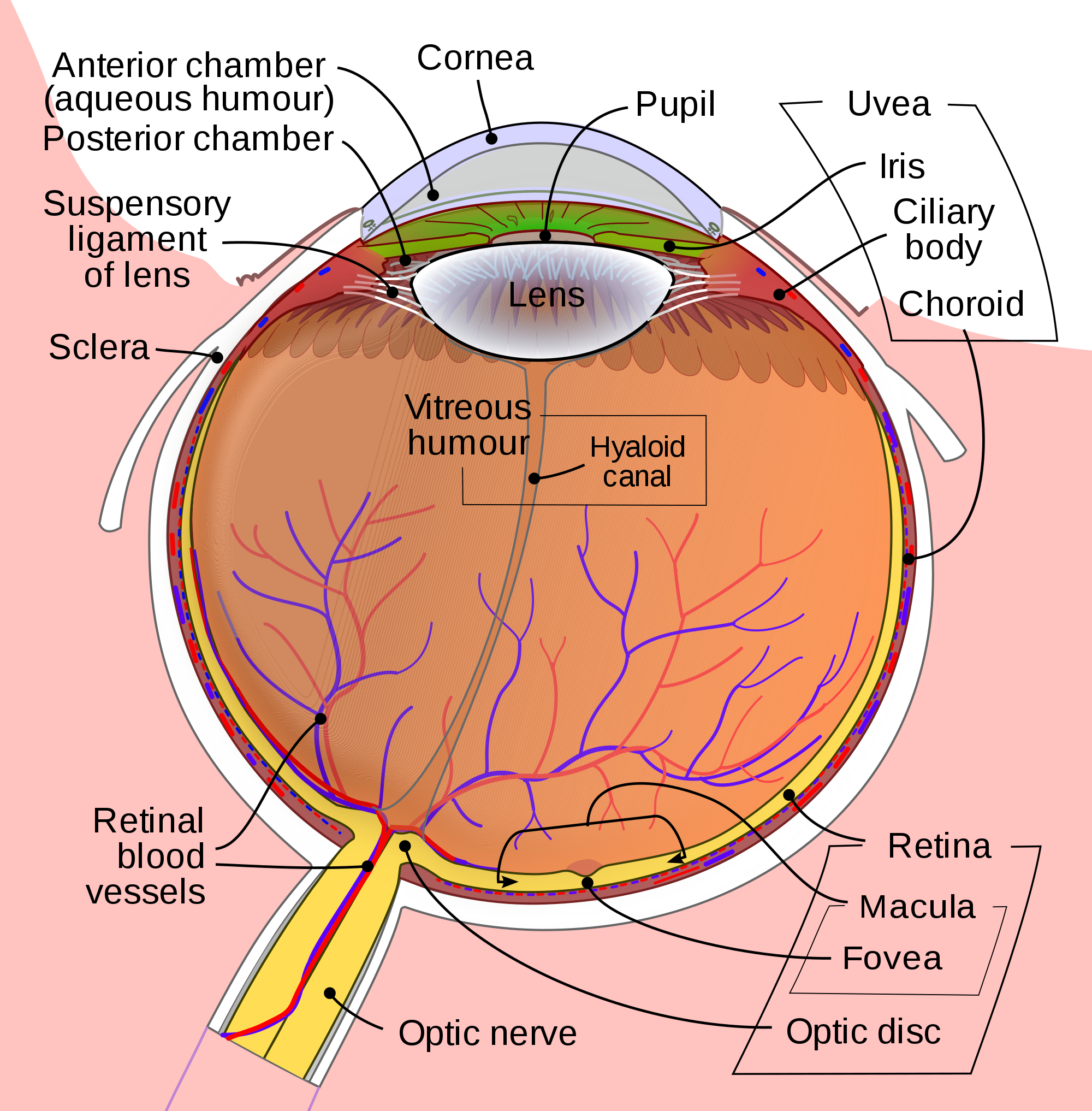
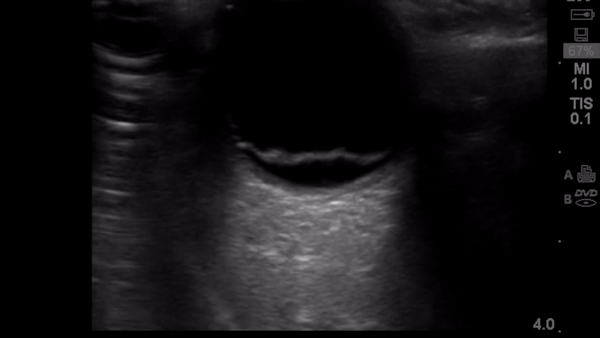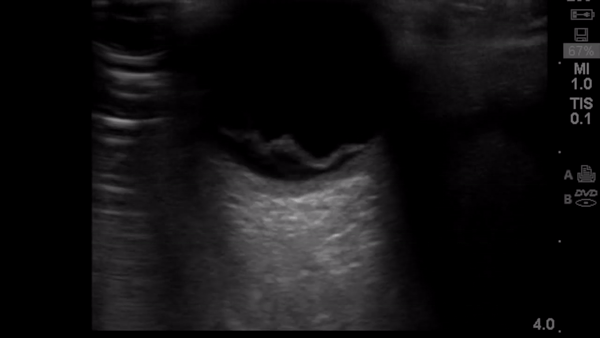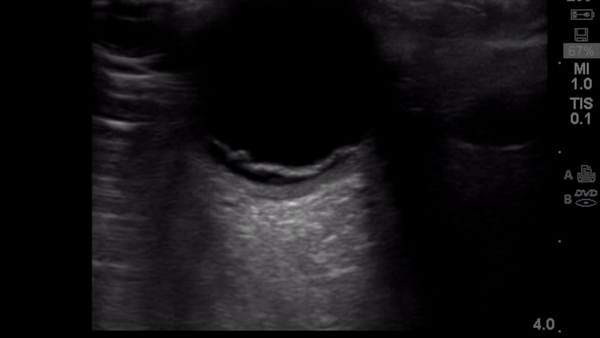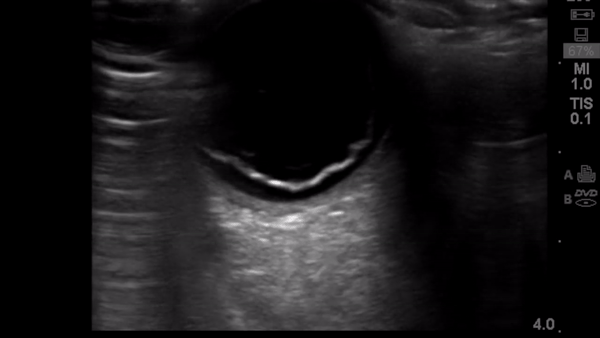
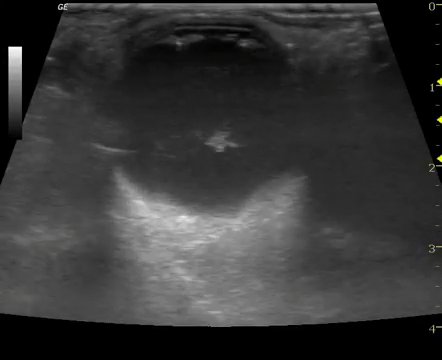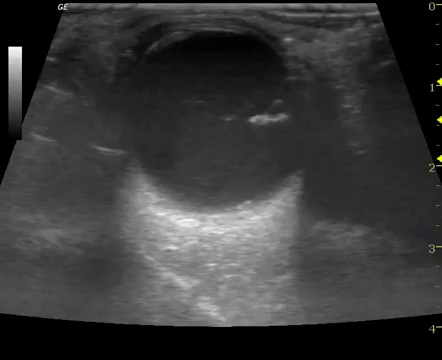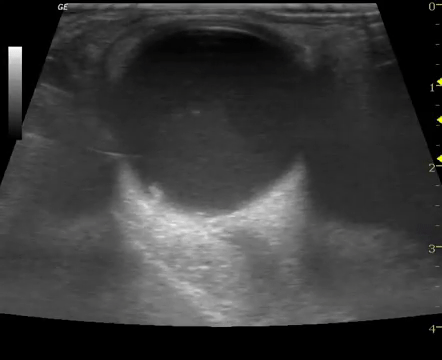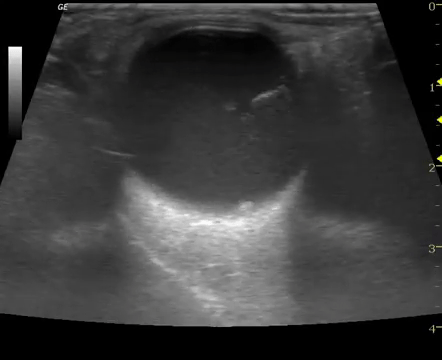
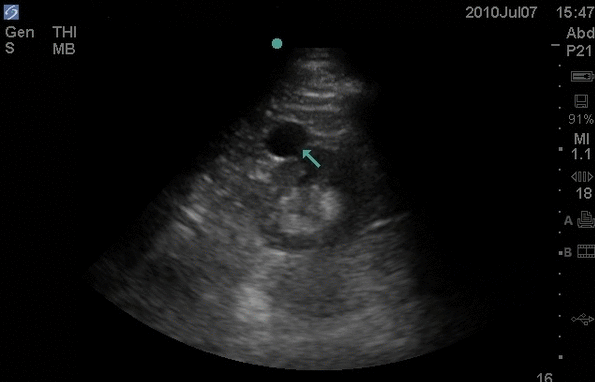
Retinal detachment (RD) is the final diagnosis for 3-4% of patients presenting to the Emergency Department (ED) with ocular complaints. Presenting symptoms most commonly include acute onset flashes and floaters, however, this presentation is not unique. The timely diagnosis and differentiation of RD from more common, benign, and similarly presenting processes, such as posterior vitreous detachment, is important in order to treat RD and prevent the sequela of permanent vision loss.
Point of care ultrasound (POCUS) has been successfully employed in the diagnosis of retinal pathology with high degrees of success according to observed test characteristics (sensitivity 97%-100%; specificity 83-100%) in emergency medicine (EM) literature. The generalizability of this data is limited, however, due to study features, including the use of highly experienced sonographers, inconsistent scanning protocols, and poor reference standards. This investigation seeks to derive the test characteristics for POCUS in the diagnosis of RD when used by a heterogeneous population of emergency physicians (EPs).
Clinical Question
What are the test characteristics (sensitivity and specificity) of POCUS for the diagnosis of RD in patients presenting with chief complaint of flashes or floaters, when performed by a group of emergency physicians with varying degrees of ultrasound experience?
Methods & Study Design
- Design
- Prospective study using a convenience sample of patients presenting to the ED with a chief complaint of flashes or floaters in visual fields
- Population
- Conducted at Vancouver General Hospital, an urban academic tertiary care center
- Inclusion Criteria
- Patients presenting with chief complaint of acute (7 days or less) onset flashes or floaters in one or both eyes between March 2015 and September 2016
- Exclusion Criteria
- Age younger than 19 years, known diagnosis of RD, exam compromised due to advanced cataract in the affected eye, ophthalmologic surgery on affected eye within prior two weeks
- Intervention
- EP performed ocular POCUS with high-frequency linear transducer
- Scan performed in both transverse and longitudinal plane with dynamic assessment of posterior chamber (patient looking left/right and up/down)
- Positive or negative interpretation for RD was recorded
- Reference Standard
- Patients were referred to an ophthalmology resident who performed non-blinded assessment including a complete dilated retinal exam
- Patients were then seen by a retina specialist blinded to the ED POCUS within 1 week, or for patients with a retinal tear or RD diagnosis, within 1 day
- Standardized training session for emergency providers
- EM attendings (20), fellows (2), and residents (8) of varying ultrasound experience received a 1 hour lecture on the use of POCUS to detect RD
- All participating EPs performed one practice scan on a healthy volunteers
- Outcomes
- Primary outcome: Accuracy of the EP diagnosis with respect to the reference standard, the retina specialist diagnosis
- Test characteristics: sensitivity, specificity, diagnostic accuracy, LR+, and LR-
Results
Flow of Patients Through Study
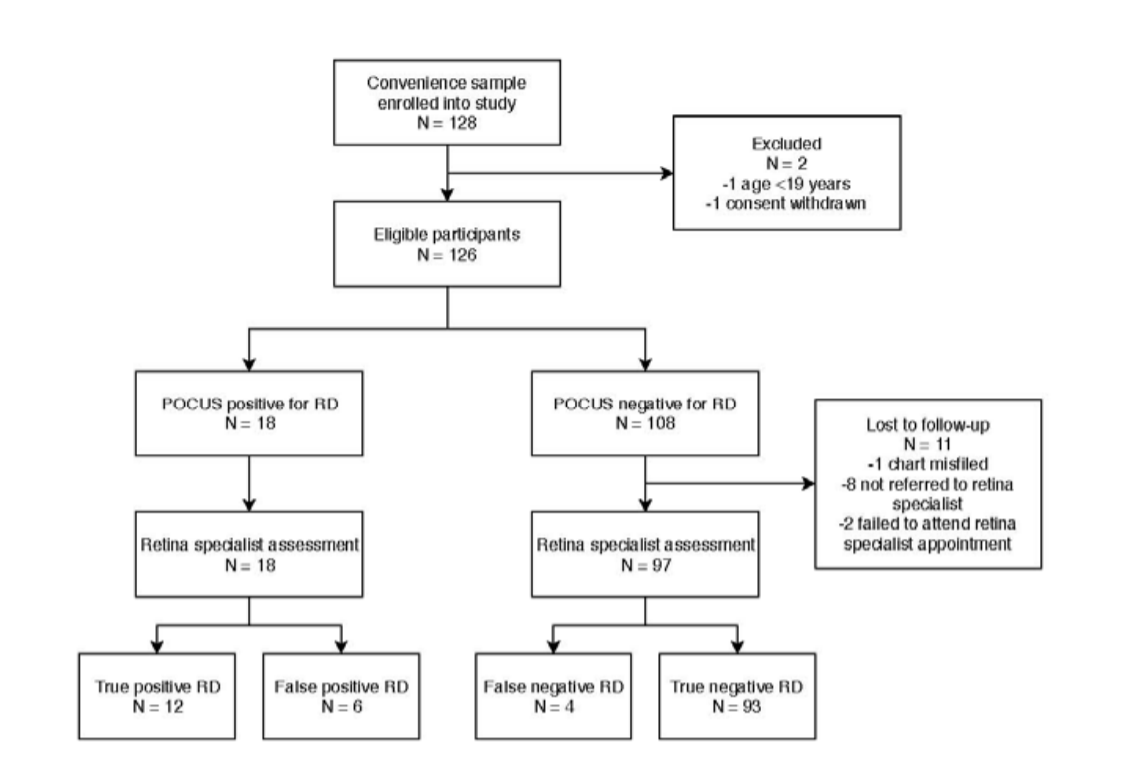 Primary analysis
Primary analysis
-
- Sensitivity: 75% (95% CI 48-93%)
- Specificity: 94% (95% CI 87-98%)
- Diagnostic accuracy: 91% (95% CI 85-96%)
- LR-positive: 12.4 (95% CI 5.4-28.3)
- LR-negative: 0.27 (95% CI 0.11-0.62)
Secondary analyses
-
- Test characteristics by level of training
- Residents and fellows: 100% sensitivity, 95% specificity
- Attending physicians: 71% sensitivity, 94% specificity
- Test characteristics by number of patients enrolled by EP
- 1-2 patients enrolled: 80% sensitivity, 71% specificity
- 3 or more patients enrolled: 73% sensitivity, 98% specificity
- Test characteristics by level of training
Limitations
-
- Insufficiently powered for the secondary analyses
- Single program study limits generalizability
- Prior ultrasound experience was not explicitly assessed
- RD is not always classically presenting, starting with a population defined by classic symptoms may influence observed test characteristics
Authors Conclusion
“In a heterogeneous group of EPs with varying ultrasound experience, POCUS demonstrates high specificity but only intermediate sensitivity for the detection of RD. A negative POCUS scan in the ED performed by a heterogeneous group of providers after a one-hour POCUS didactic is not sufficiently sensitive to rule out RD in a patient with new onset flashes or floaters.”
Our Conclusion
This study demonstrates that emergency physicians of varying training levels and ultrasound experience can successfully employ POCUS in the diagnosis of RD after only a short training session. By incorporating POCUS into the workup of patients presenting with ocular complaints characteristic of RD, true pathology can be identified with high specificity. Appropriate care can then be mobilized expeditiously in these scan-positive patients in order to prevent the permanent vision loss associated with this condition.
Indeed, a 74% sensitivity is too low for POCUS to reliably be utilized by a heterogeneous population of EPs as a tool to rule-out RD, especially given the consequences of a missed diagnosis. It would be reasonable practice, therefore, as the authors suggest, for all patients with new onset flashes and/or floaters to continue be referred for further ophthalmologic evaluation to definitively rule-out RD and other conditions at-risk for progression to RD. It should also be noted, however, that a trend towards increased specificity was observed amongst physicians who enrolled more patients in this study. Taken in context with test characteristics reported in prior literature, these findings may suggest that specificity can be improved upon with experience, and in the hands of a trained sonographer, POCUS may also be used as a tool to reliably rule-out RD.
The Bottom Line
Emergency providers can reliably use point-of-care ultrasound to diagnose retinal detachment with high specificity after a short, one-time training course, but must recognize the limitations of POCUS as a tool to rule-out RD in this setting, given a relatively low sensitivity when used for this purpose.
Authors
This post was written by Oretunlewa Soyinka, MS4 at UCSD. Review and further commentary was provided by Cameron Smyres, MD, Ultrasound Fellow at UCSD.
References
1 . Hikichi T, Hirokawa H, Kado M, et al. Comparison of the prevalence of posterior vitreous
detachment in whites and Japanese. Ophthalmic Surg 1995; 26:39-43.
2. Hollands H, Johnson D, Brox AC, et al. Acute-onset floaters and flashes: is this patient at
risk for retinal detachment? JAMA 2009; 302:2243-9
3. Alotaibi AG, Osman EA, Allam KH, et al. One month outcome of ocular related
emergencies in a tertiary hospital in Central Saudi Arabia. Saudi Med J 2011; 32:1256-60.
4. Mitry D, Charteris DG, Fleck BW, et al. The epidemiology of rhegmatogenous retinal
detachment: geographical variation and clinical associations. Br J Ophthalmol 2010;
94:678-84.