A previously healthy 28-year-old male presents to the Emergency Department complaining of one month of fatigue, shortness of breath, and dyspnea on exertion. These symptoms were preceded by symptoms of a viral illness which initially improved; however, he had recurrence of symptoms two weeks ago. He was seen at urgent care five days ago and was given steroids and albuterol without improvement. The patient otherwise denies any infectious symptoms, leg swelling, or risk factors for pulmonary embolus or deep vein thrombosis.
VS: T: 97.7F BP: 129/87. HR: 109 RR: 16. SP02: 95%
Patient is alert and oriented, non-toxic in no distress, and behaving appropriately. Cardiac exam shows a RRR, no murmurs, rubs, or gallops. Lung exam is consistent with shallow breaths and dyspnea with conversation, otherwise lungs are CTAB with no wheezing, rales, or rhonchi. The patient has no chest wall tenderness, no JVD, and no lower extremity edema.
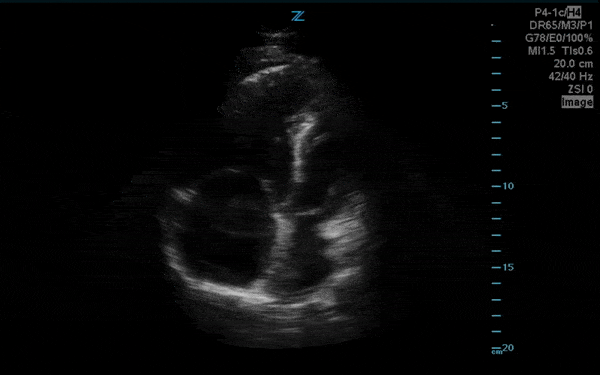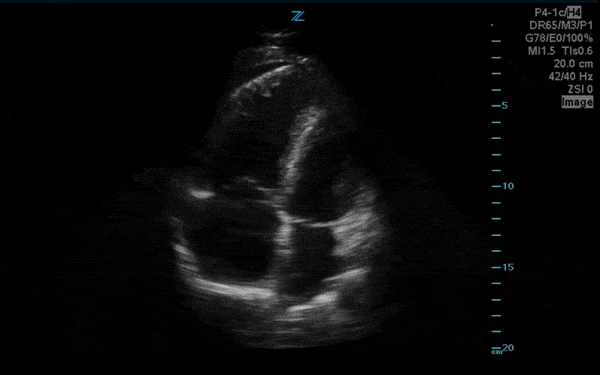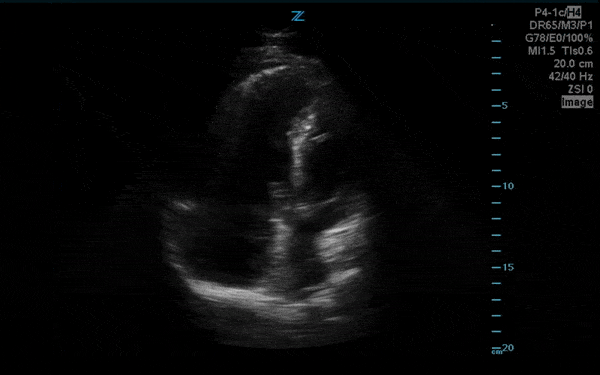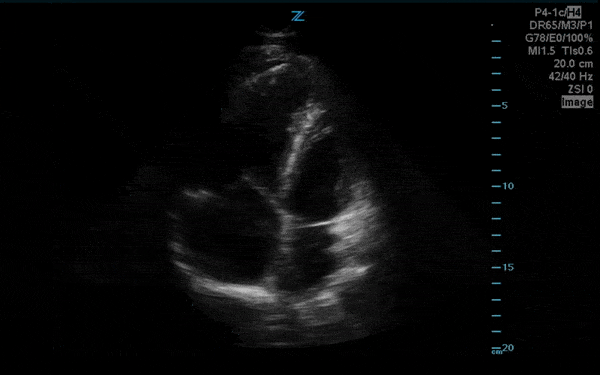
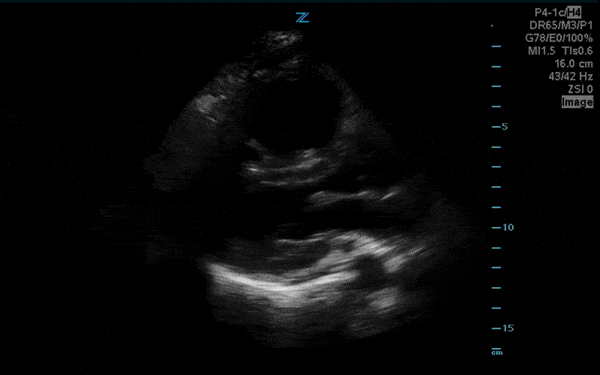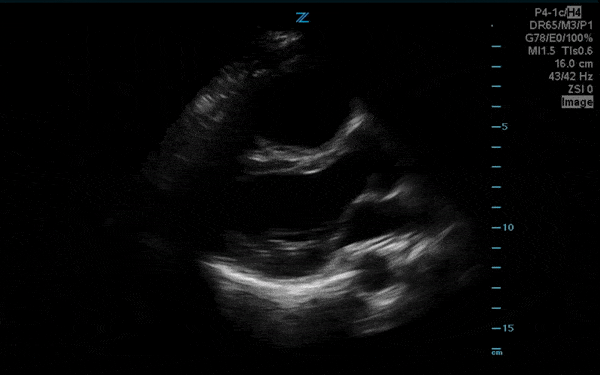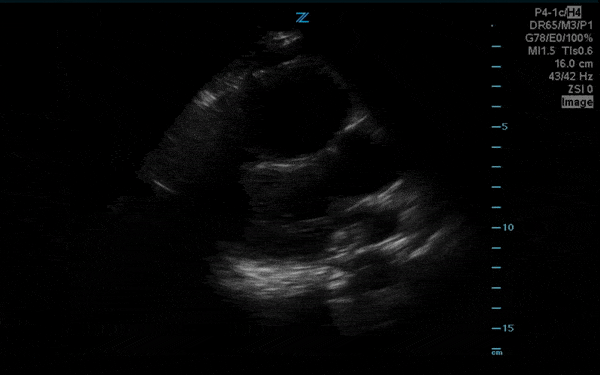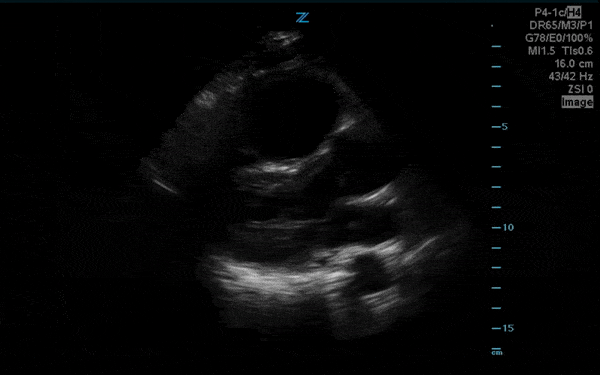
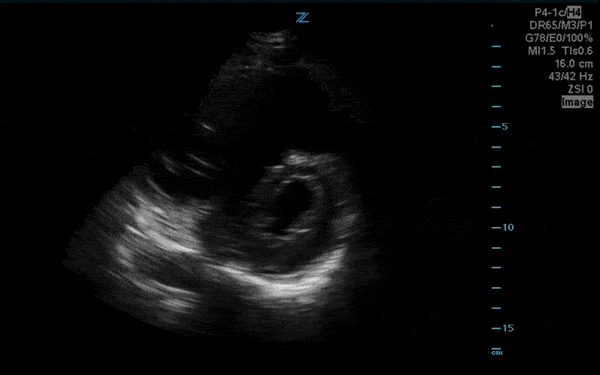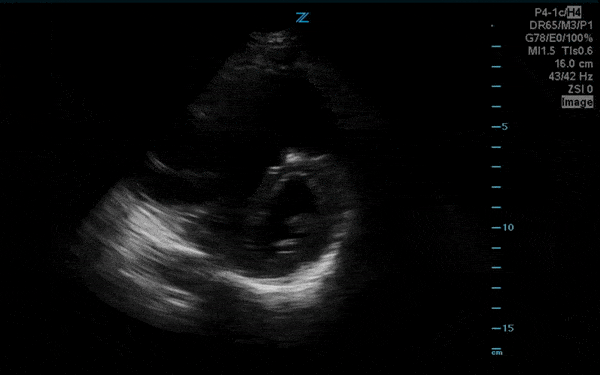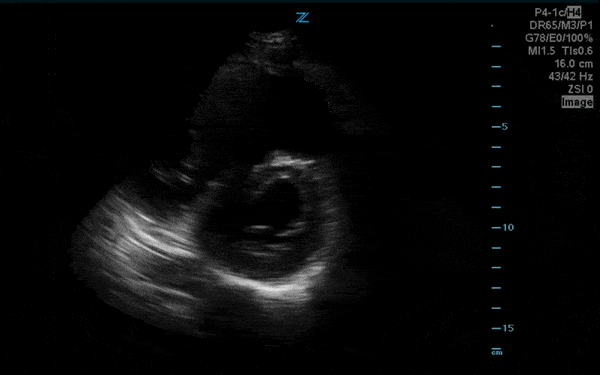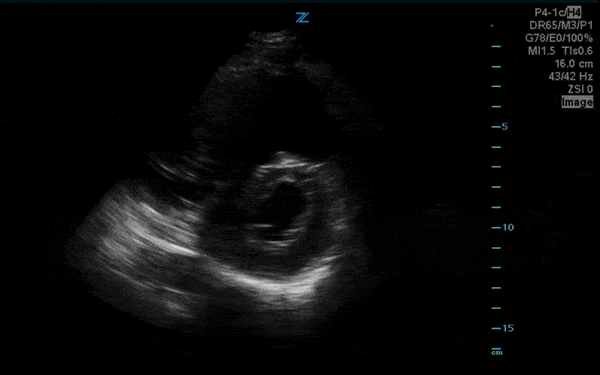
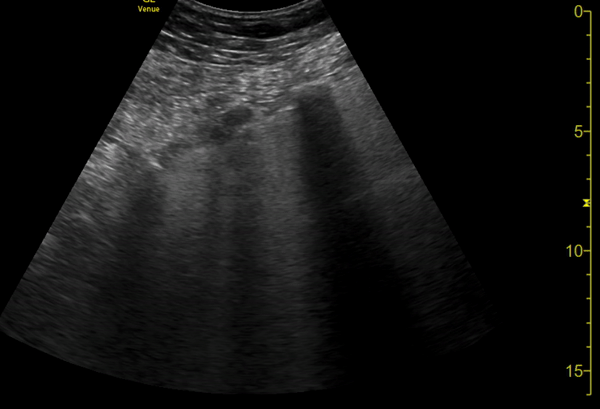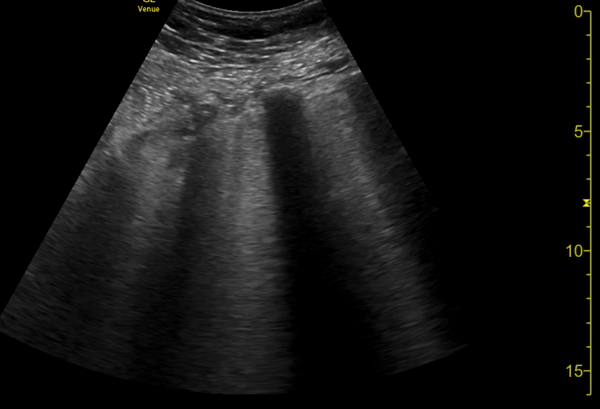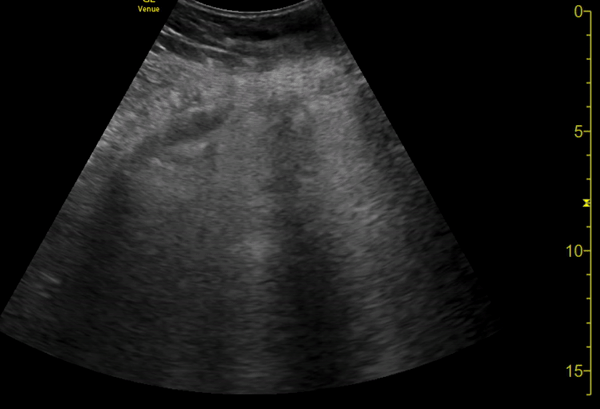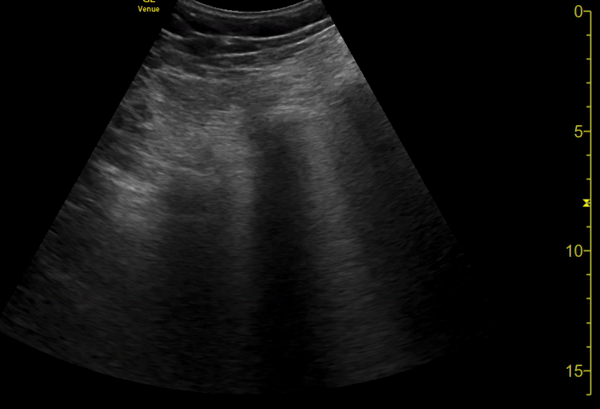
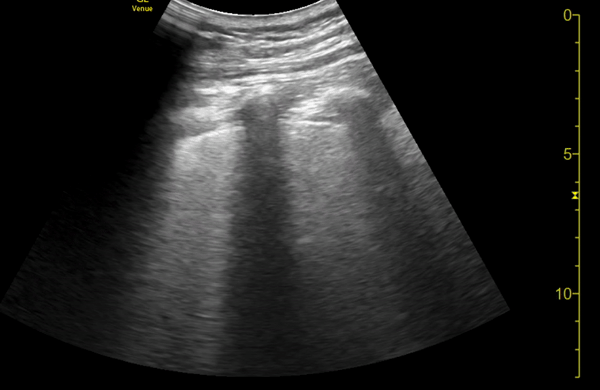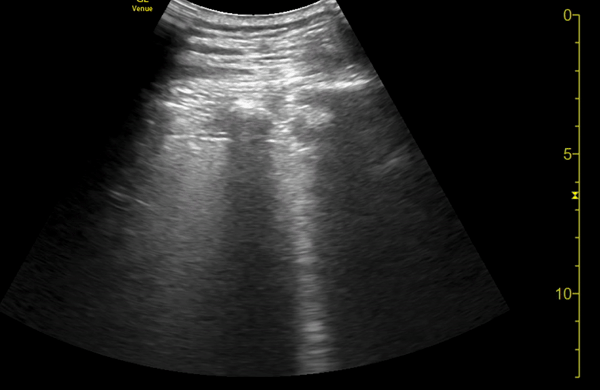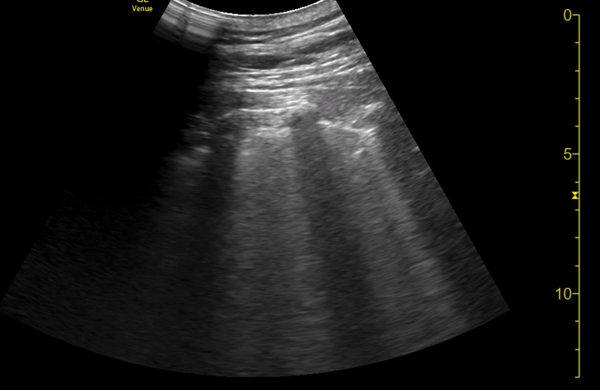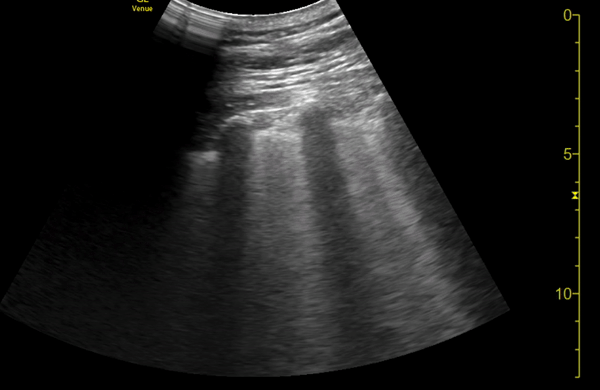
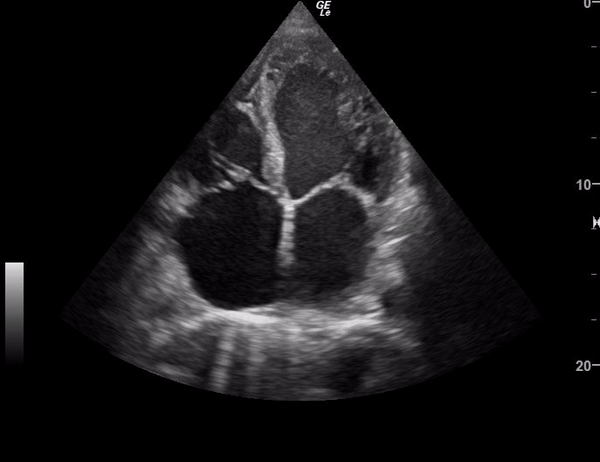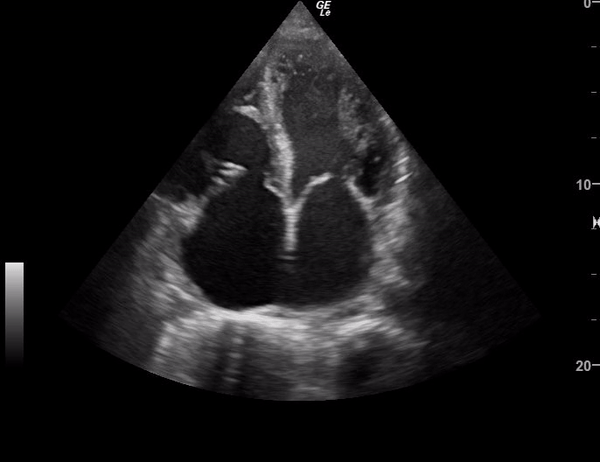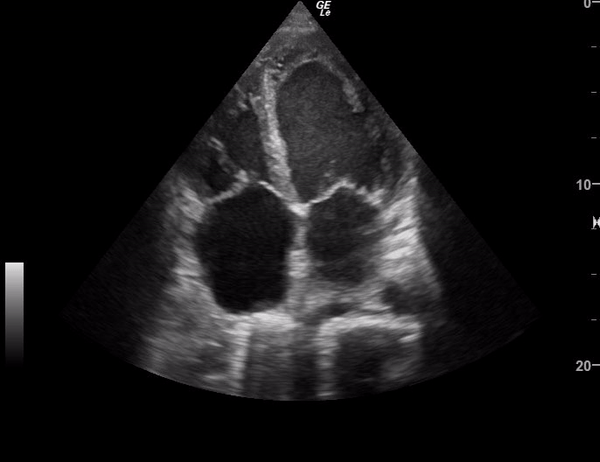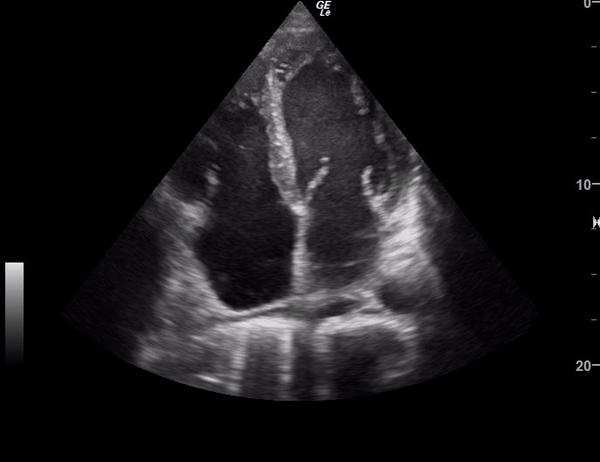
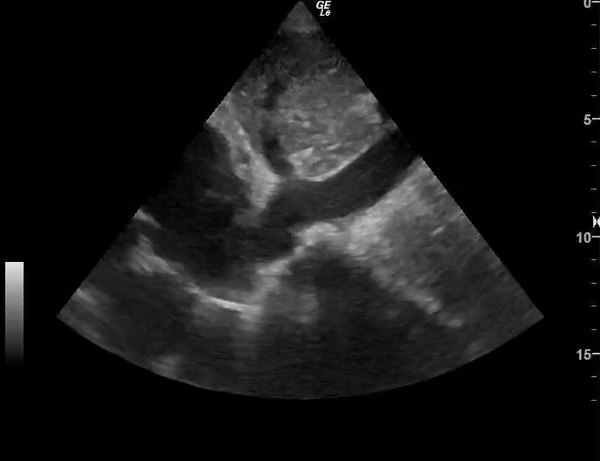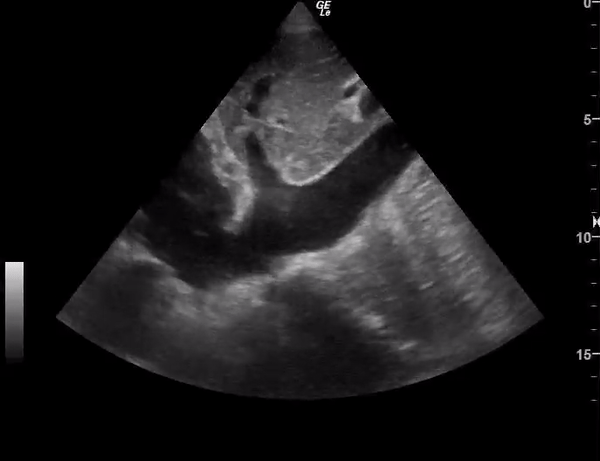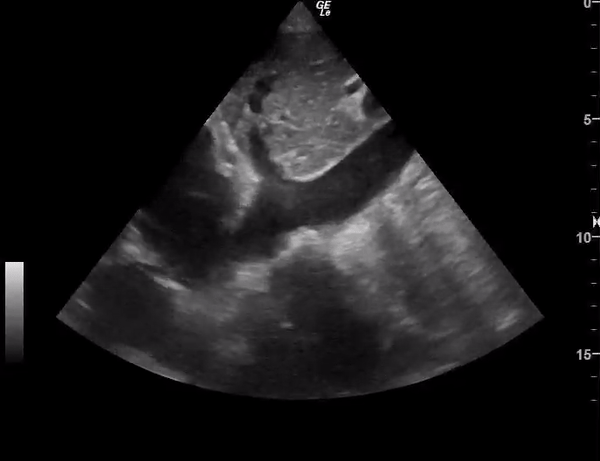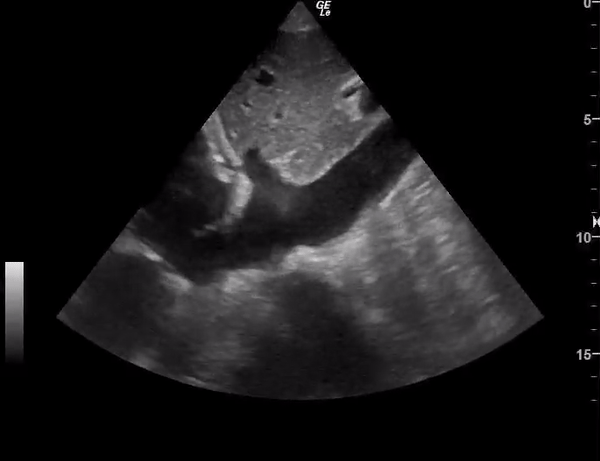
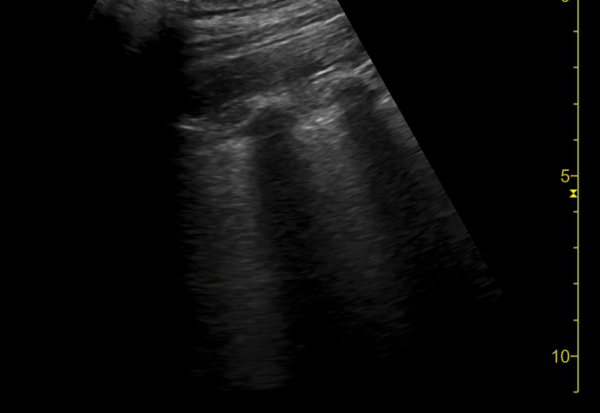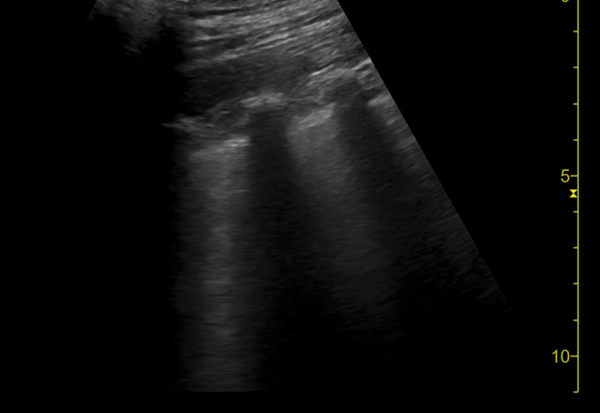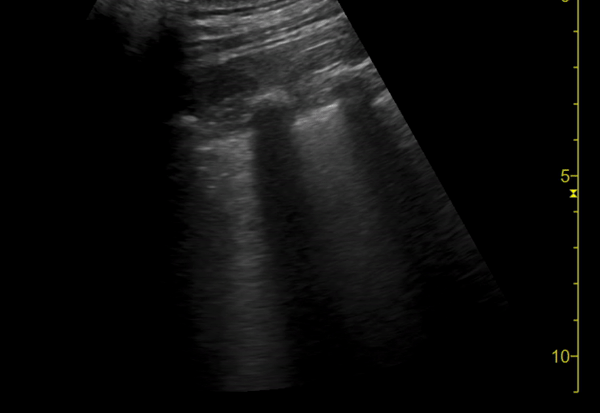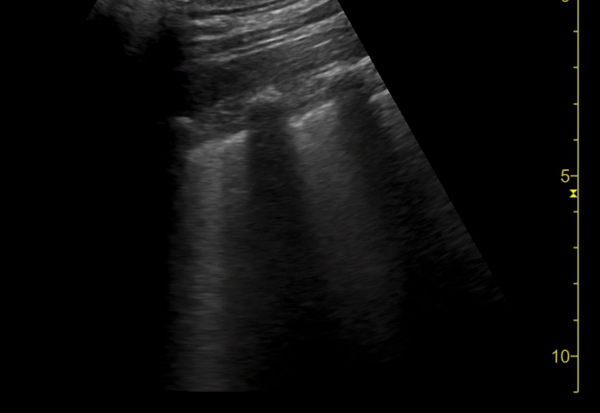
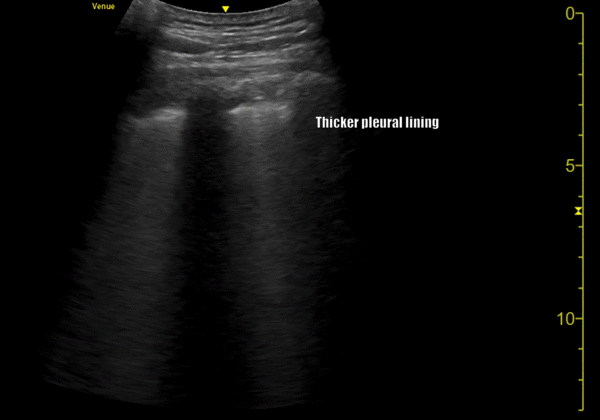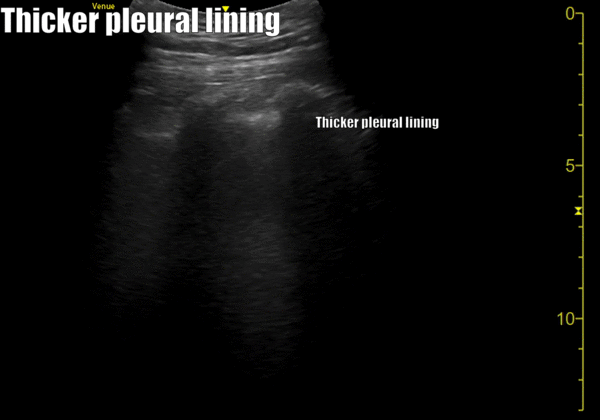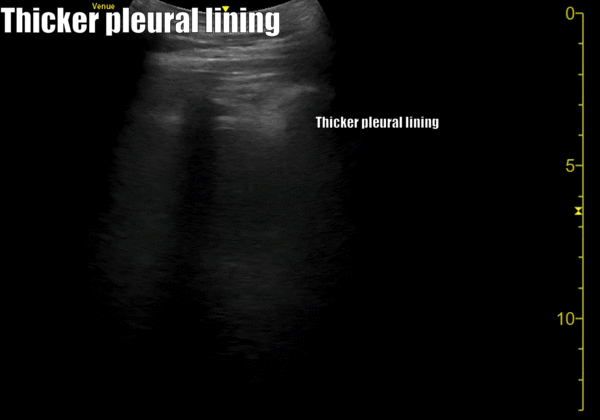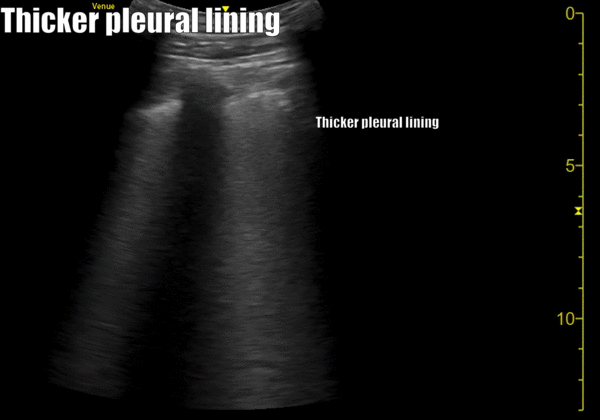
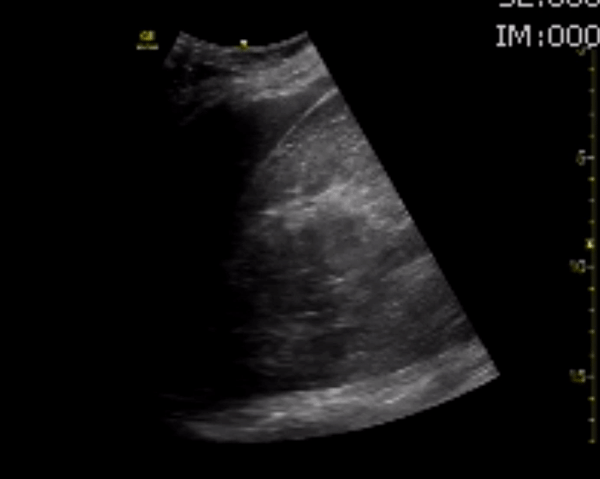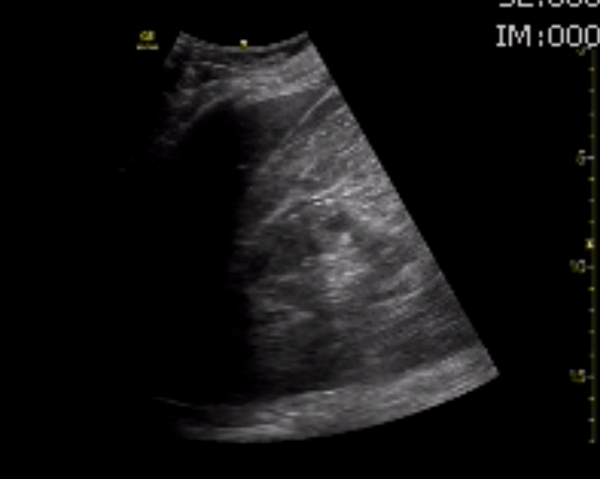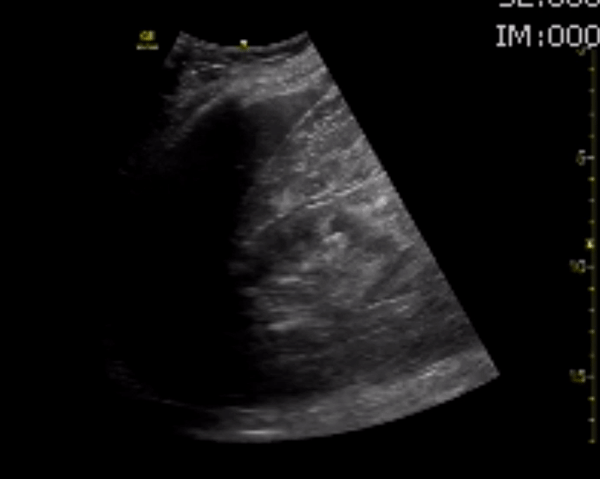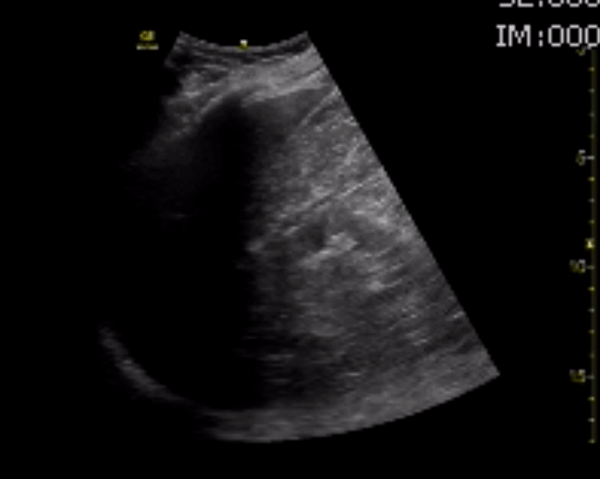
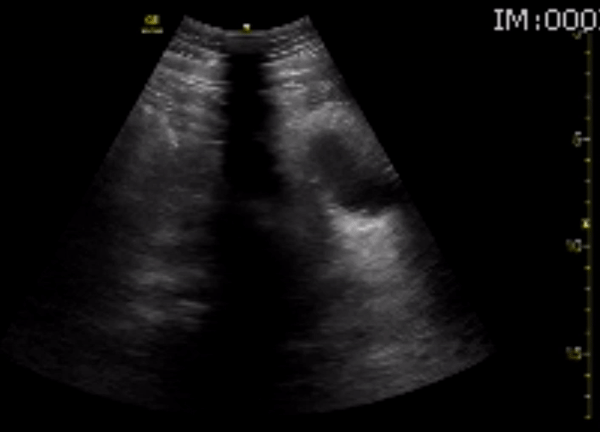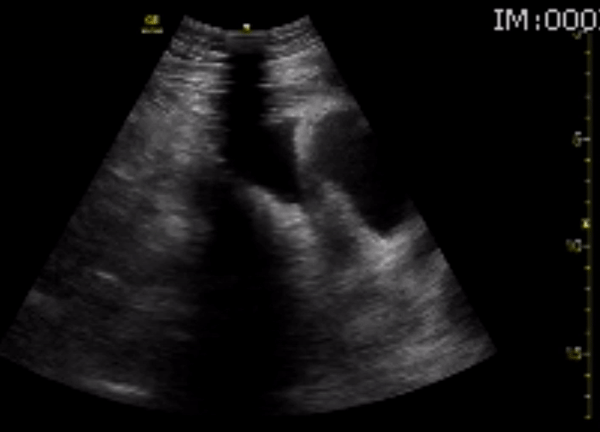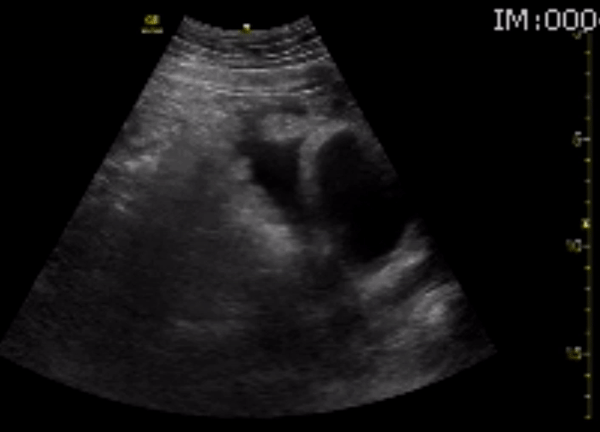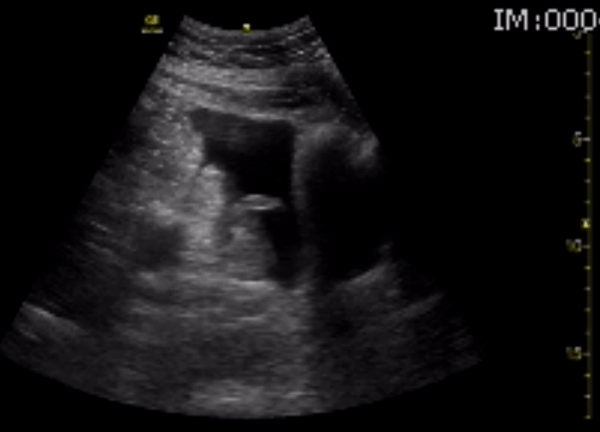
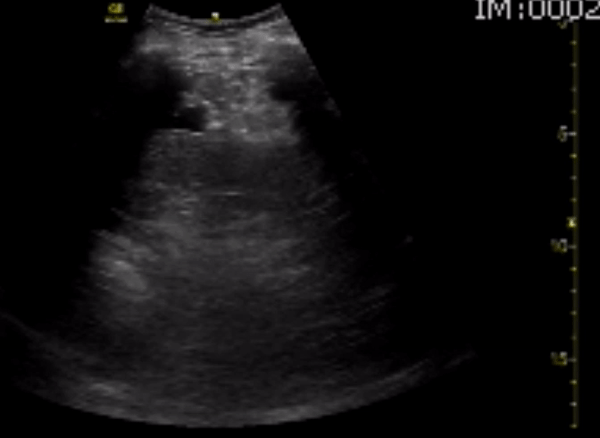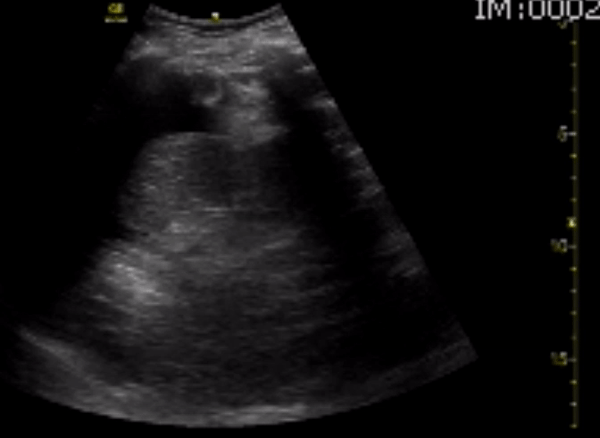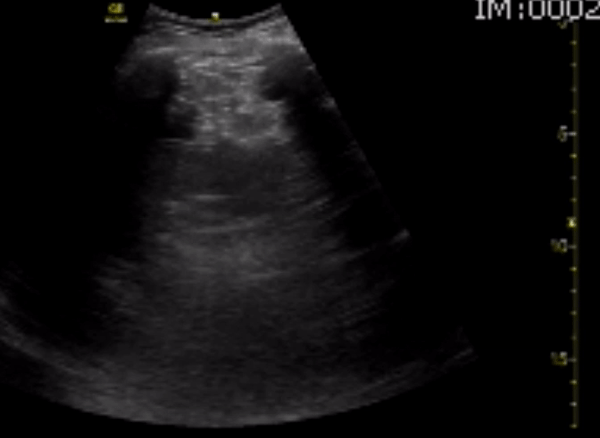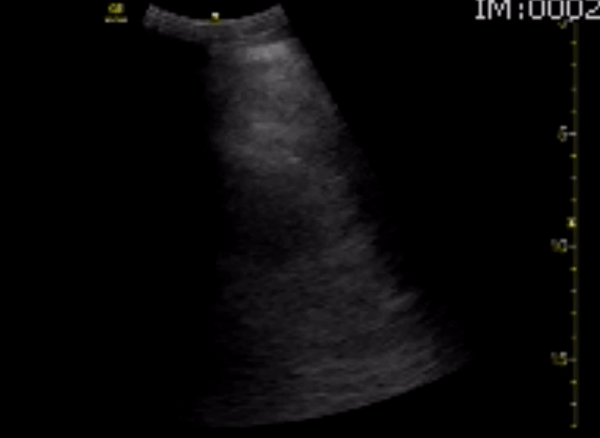
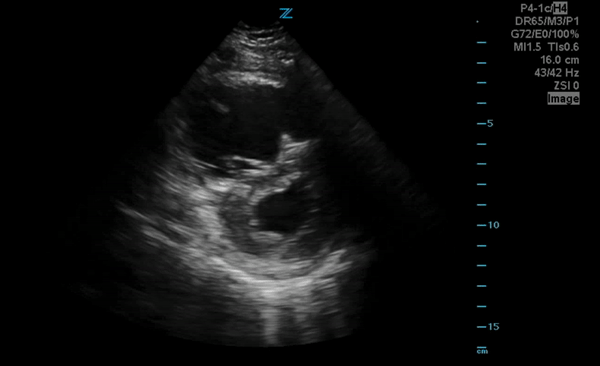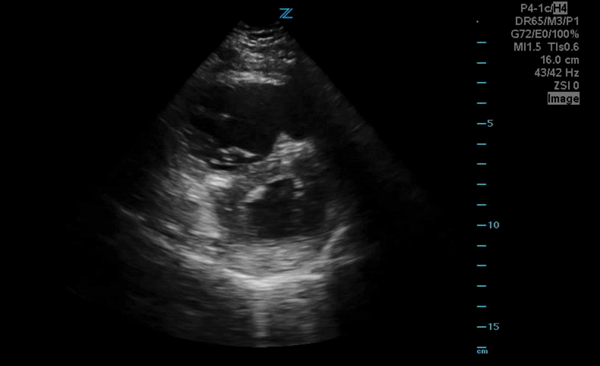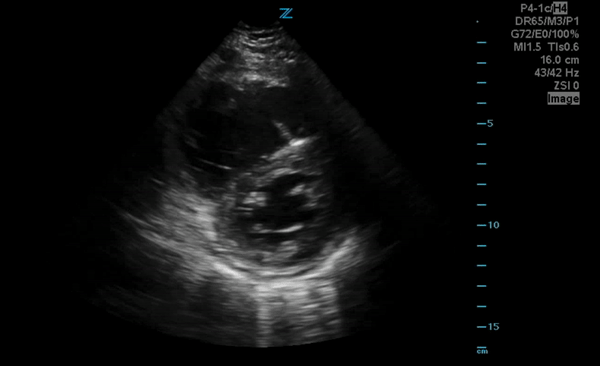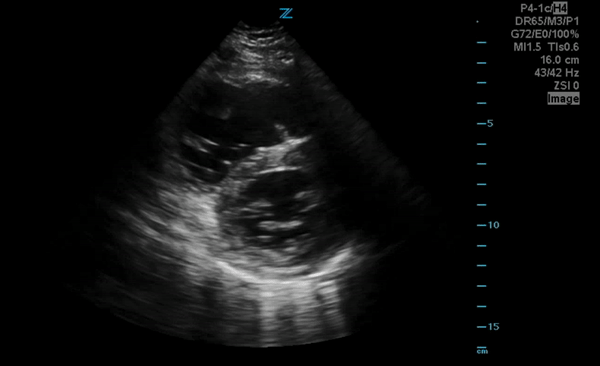
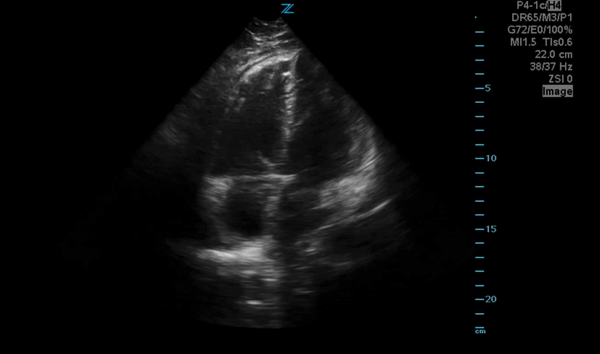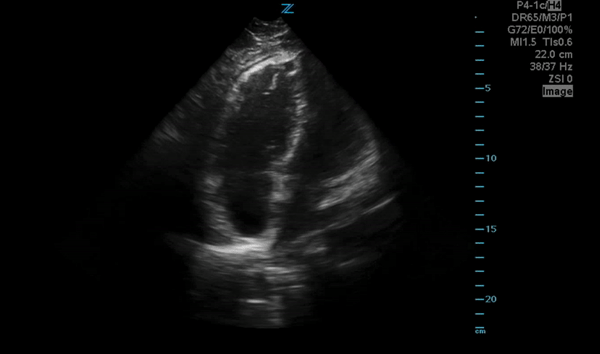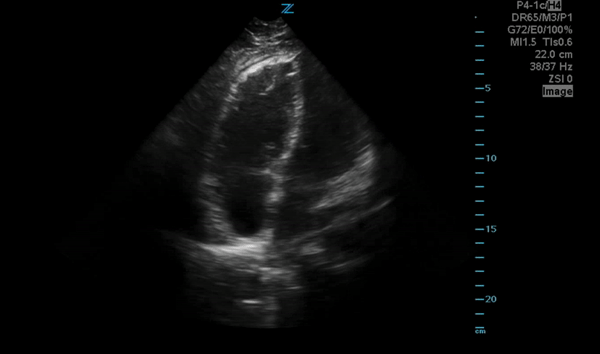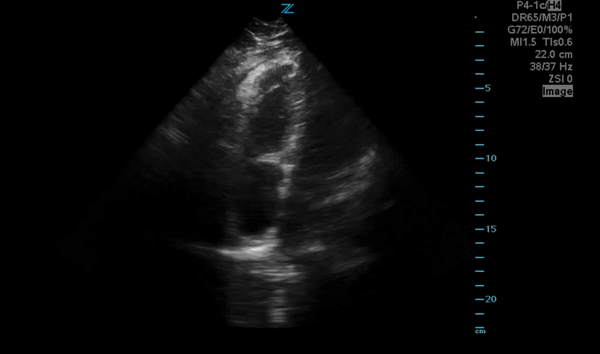
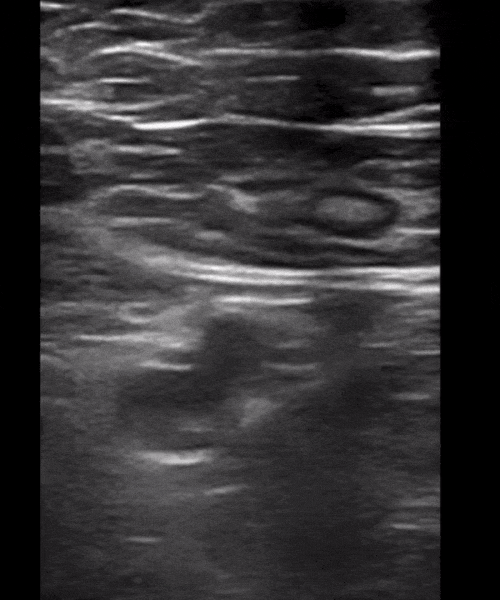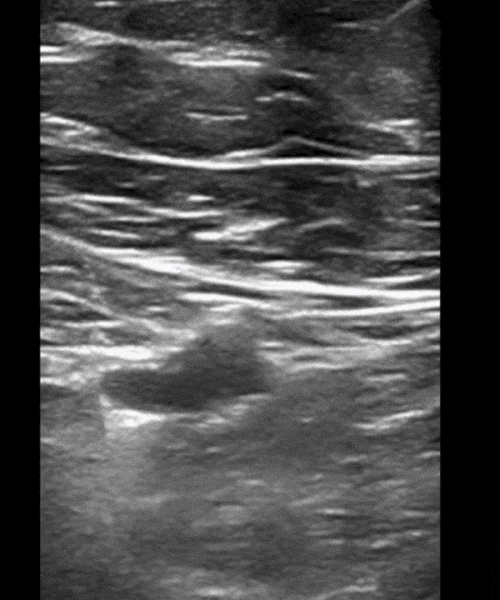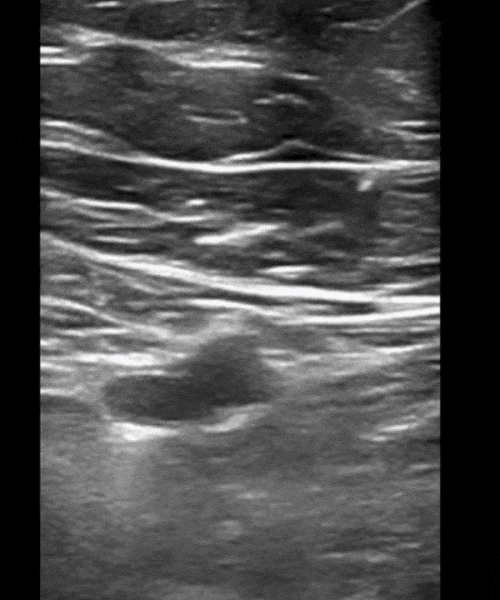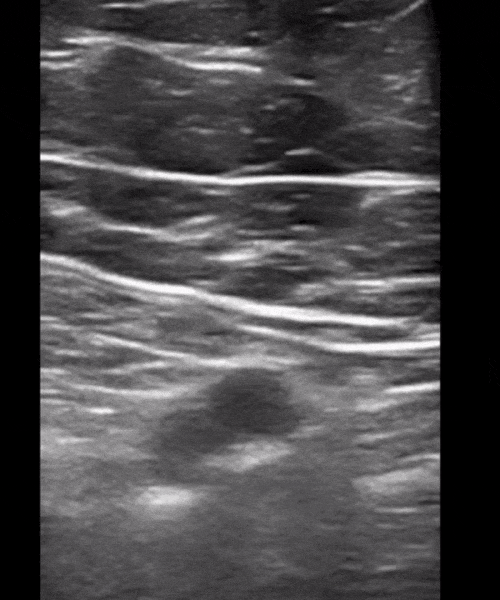
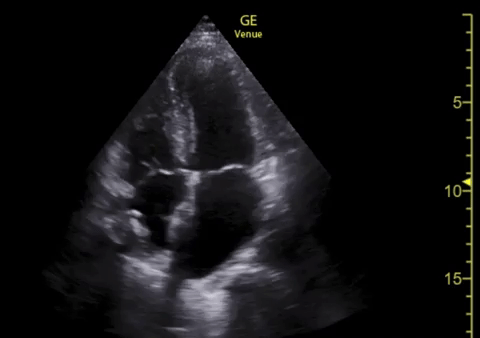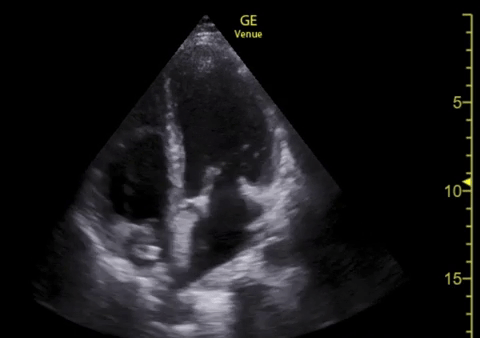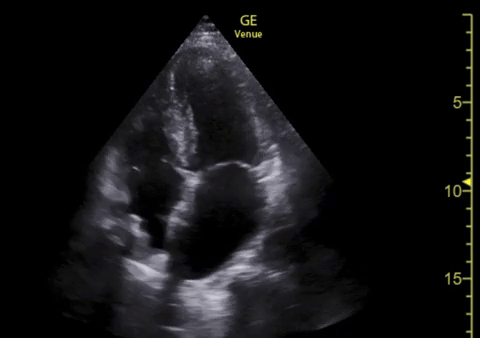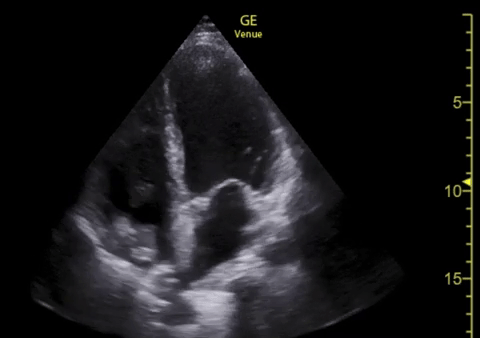
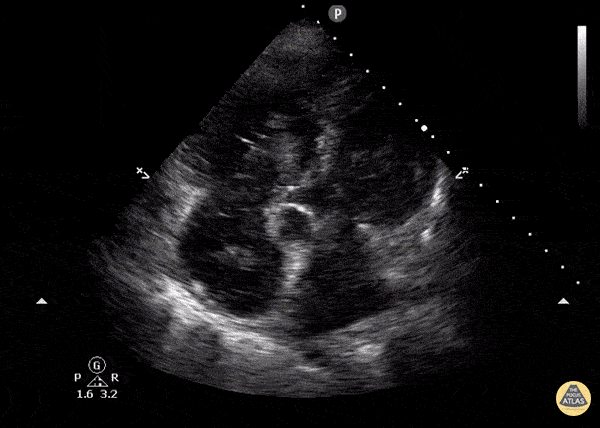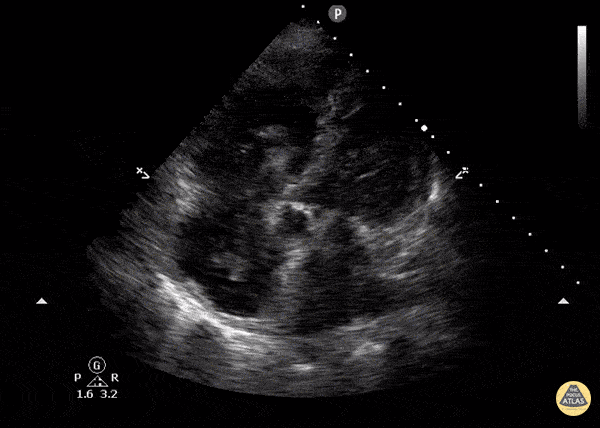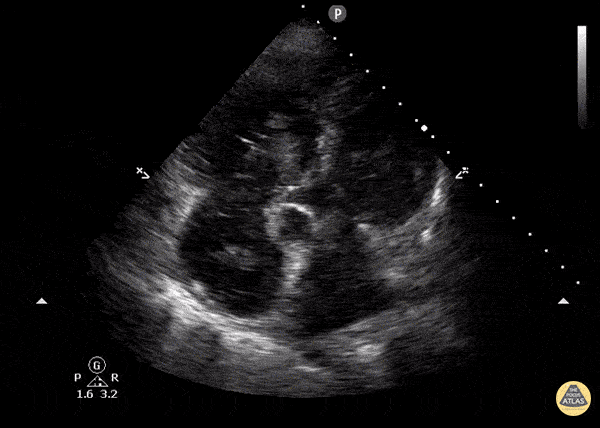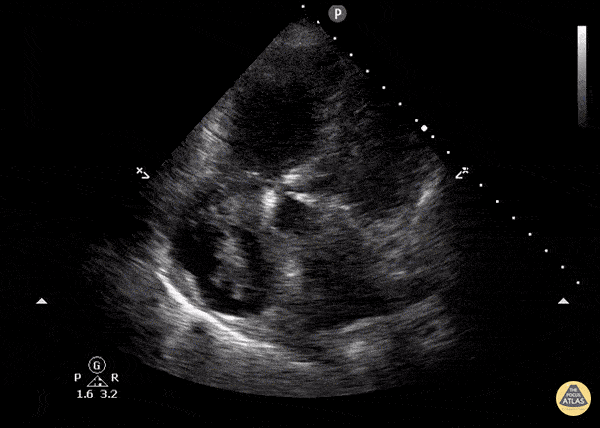
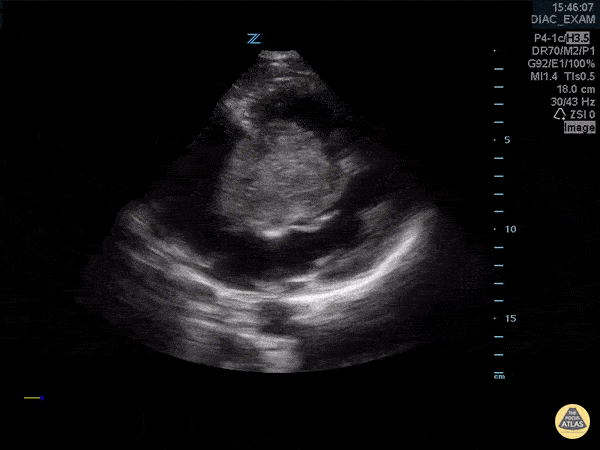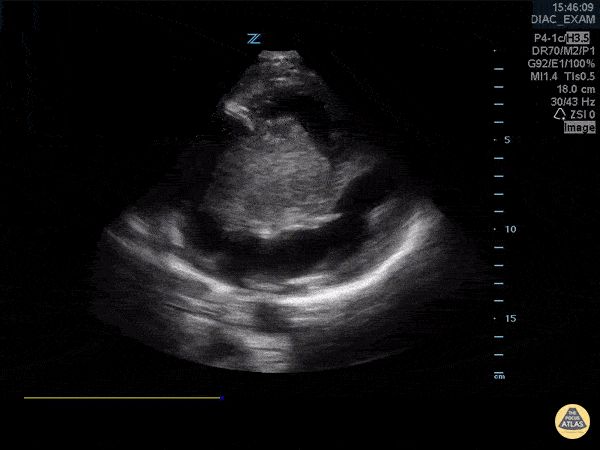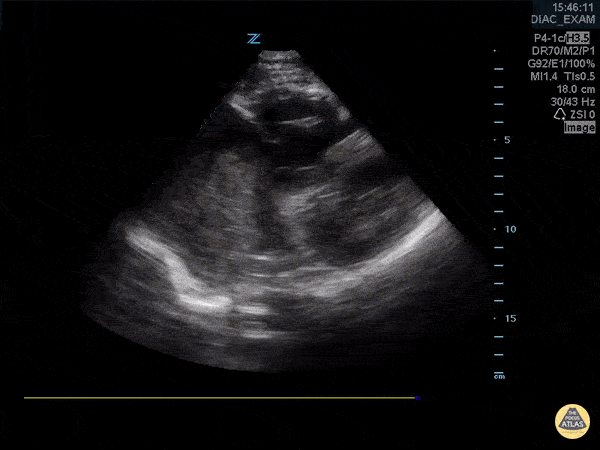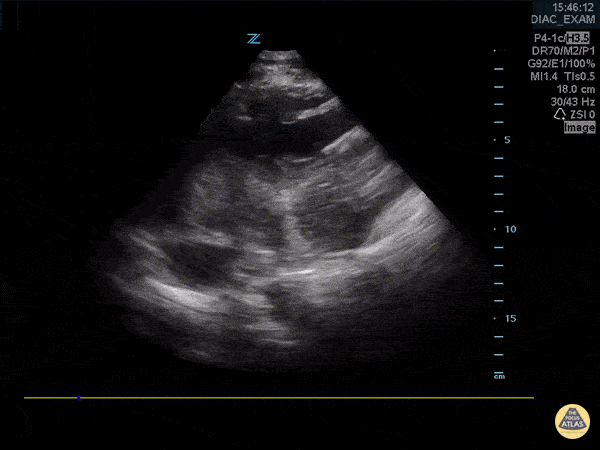
You perform a bedside ECHO and you see the following. What do you see and what is your most likely diagnosis? What is your next step in management?
Answer and Learning Points
Answer:
In all three cardiac views, there is dilation of the right side of the heart. In the parasternal short axis you see septal bowing into the left side of the heart, also known as the “D” sign (named after the shape of the left ventricle). These findings are indicative of elevated right sided pressure, or right heart strain, which can be seen in conditions such as acute pulmonary embolism, pulmonary hypertension, COPD, and right ventricular infarction. Given the relatively thin free wall of the right ventricle, the likely cause of right heart strain in the above scenario is an acute process.
The patient had a CT scan that revealed extensive pulmonary emboli in all segmental and subsegmental arterial divisions of the lung with findings consistent with pulmonary artery hypertension and severe right heart strain. The EKG obtained had evidence of right heart strain including right axis deviation and diffuse T-wave inversions. The patient was started on heparin and admitted to the ICU.
Learning Points
- The reported sensitivity and specificity of echocardiography in demonstrating right heart dysfunction are around 56% and 42% respectively (1)
- Described features of right heart dysfunction include (2)
- Dilation of the right ventricle
- The RV normally appears triangular-shaped and is two-thirds the size of the LV in apical four view (3)
- Dilation of the right ventricle
-
- Interventricular septal flattening
-
-
- AKA the “D sign” on parasternal short view or paradoxical septal motion on apical four view
-
-
- Right ventricular hypertrophy (right ventricular free wall thickness >5mm in diastole)
-
-
- When present, implies some degree of chronicity to the inciting hemodynamic insult
-
-
- Right ventricular hypokensia
-
-
- Typically quantified as a tricuspid annular plane systolic excursion, as measured by M-mode from the apical 4 chamber view, <1.6 cm
-
-
- Plethoric vena cava
References
- He, H., et-al. Computed tomography evaluation of right heart dysfunction in patients with acute pulmonary embolism. J Comput Assist Tomogr. 2006;30 (2): 262-6.
- Rudski, L.G., et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. (2010) Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 23 (7): 685-713
- Mallin, M, Dawson, M. Introduction to Bedside Ultrasound: Volume 2. Emergency Ultrasound Solutions, 2013. Apple Books. https://books.apple.com/us/book/introduction-to-bedside-ultrasound-volume-2/id647356692. Accessed April 17th, 2020.
The following authors contributed to this post:
Danika Brodak, MD; Amir Aminlari, MD; Rachna Subramony, MD; Colleen Campbell, MD









 Adhikari, S. R. (2008). Small parts - Testicular ultrasound. Retrieved from
Adhikari, S. R. (2008). Small parts - Testicular ultrasound. Retrieved from  Prando D.
Prando D.