Background
Traditionally, the focus of emergency point-of-care echocardiography has been threefold: to assess left ventricular systolic function, to identify the presence of right ventricular enlargement and to evaluate for pericardial effusion. Assessing for regional wall motion abnormalities has been left to formal echocardiography and Cardiology (1). However, structural abnormalities can appear within seconds from the onset of myocardial ischemia (2), so identifying wall motion abnormalities in patients with chest pain or suspected acute coronary syndrome (ACS) in the Emergency Department may be clinically beneficial for emergency providers, leading to more prompt and appropriate diagnostic or therapeutic measures.
We evaluate the following article that looks at whether ED physicians can accurately identify regional wall motion abnormalities.
Clinical Question
Can emergency physicians with basic training in emergency echocardiography accurately identify regional wall motion abnormalities (RWMA) in patients admitted with STEMI?
Methods & Study Design
• Design
Observational report – one group of residents trained and tested in an ultrasound procedure.
• Population
75 patients admitted with STEMI. 6 were excluded from the analysis due to withdrawal, leaving AMA or inability to obtain interpretable images.
• Intervention
Nine residents viewed 2 video instructional modules to provide an introduction to identifying RWMA, and completed an online test evaluating echocardiographic clips for RWMA. They then performed a bedside echocardiogram on patients with known STEMI, though they were blinded to any clinical data about the patient, including the EKG. This was performed within 24 hours of the formal comprehensive echocardiogram.
• Outcomes
The primary outcome was agreement between resident performed echo and formal comprehensive echo on the presence and localization of RWMA.
Results
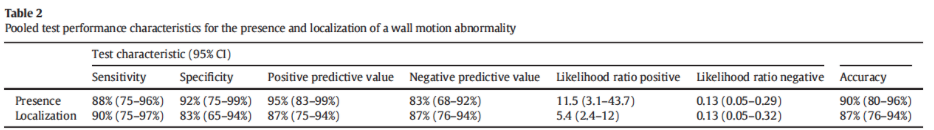
62% of subjects enrolled had a wall motion abnormality identified by the reference standard. Study investigators identified the presence of RWMA with good sensitivity and specificity (Table 2).
Inter-rater agreement between the point-of-care echocardiogram and the formal echocardiogram for the presence of RWMA was K = 0.79 (95% CI: 0.64–0.94).
Strength & Limitations
Strengths:
Promising results suggest that emergency medicine physicians can be taught to accurately identify RWMA in STEMI with little training. Though the patients in this study were already known to have STEMI on EKG, the application of this procedure may be helpful when patients arrive with NSTEMI or elevated cardiac markers to help in the clinical decision making.
Limitations:
Study was conducted using just 9 residents, and 2 of the residents did the vast majority of the scans. It is possible that these residents are already skilled in ultrasound, so to truly gauge whether this method is broadly teachable, many more residents (with varying levels of baseline ultrasound experience) would need to be evaluated.
Authors Conclusion
The ability to diagnose a RWMA offers emergency clinicians another tool to help manage patients with chest pain and suspected ACS. These data support the introduction of focused training in RWMA identification and expansion of the clinical use of emergency and critical care echocardiography.
Our Conclusion
This is an interesting concept that emergency medicine residents can be trained to successfully identify RWMA using echocardiography. If, and how, this should be implemented in clinical practice is still yet to be explored. Perhaps this could be used in cases of NSTEMI or elevated cardiac markers to help inform clinical decision making, but this study does not answer the question of whether this skill will be clinically useful for ED physicians.
The Bottom Line
It is possible to train emergency medicine physicians to identify regional wall motion abnormalities using echocardiography.
Authors
This post was written by Allison Auchter, MS4 at UCSD School of Medicine, Charles Murchison, MD and Amir Aminlari, MD.
References
P.E. Croft, T.D. Strout, R.M. Kring, et al., WAMAMI: emergency physicians can accurately identify wall motion abnormalities in acute myocardial infarction, American Journal of Emergency Medicine.
-
-
Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines of the Clinical Application of Echocardiography). Circulation 2003;108(9):1146–62.
-
Wholgelernter D, Cleman M, Highman HA, et al. Regional myocardial dysfunction during coronary angioplasty: evaluation by two-dimensional echocardiography and 12 lead electrocardiography. J Am Coll Cardiol 1986;7(6):1245.
-